The memories of our beloved pets have a ripple effect throughout our lives forever. We will always look back and reminisce about all the laughs and love they brought to our lives. These memories include the way we choose to say goodbye.
A professional service that shows respect to our pets and our planet
At Ripple, we offer Aquamation services to help return your beloved pet’s to nature after they have passed away.
Aquamation, also known as ‘Water Cremation’ or ‘Alkaline Hydrolysis’, allows you to say goodbye to your pet in a way that respects their memory and the environment.
Aquamation has been around for decades and is fast becoming the preferred choice for pet owners worldwide.
For some of us, knowing the details and intricacies of how something works brings us comfort.

What is the process?
The process of Aquamation is much more sustainable than cremation or burial. The method uses water instead of fire and mimics the way bodies are returned to nature in the wild.
Fire cremation utilised temperatures between 800-1000 Celsius, which requires a considerable amount of energy. Also, every fire cremation releases about 200kg of greenhouse gases, compared to Aquamation, where approximately 20kgs are released.
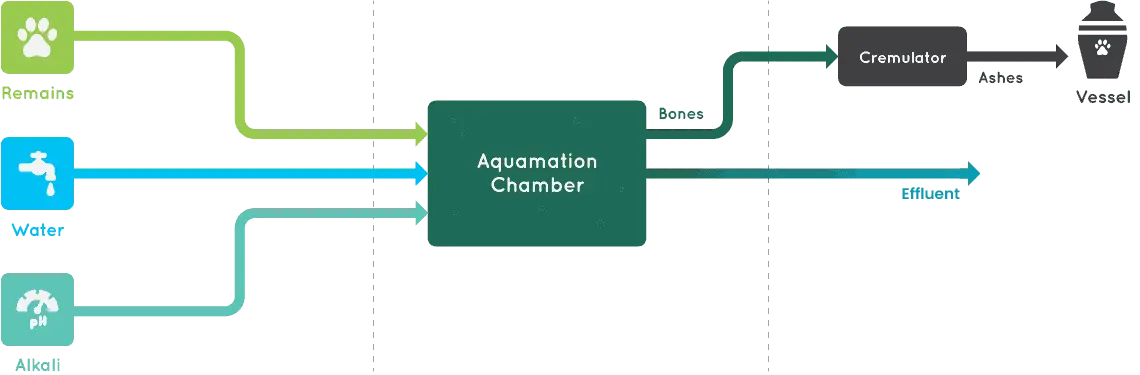
Step1
The body is placed in a chamber with a combination of warm water and alkali; a dissolvable salt obtained from the remains of plants and made up mostly of potassium and sodium carbonate. Alkali has properties that help things break down in nature.
Step2
The solution works to accelerate the natural occurrence of hydrolysis, rapidly breaking down all the tissue, except the ones. This is the same as would occur in mother nature, through oxidation, just simply at a faster rate.
Step3
Aquamation does not destroy the bone matter, and after the process is complete, (much like with flame cremation), the bones are dried, placed into a cremulator and turned into a powder (ash) which can then be kept in an urn or scattered.
Need to know more about the Aquamation process? Here is another good explanation from Alan Hillsberg
